This book is ideal for who want to use a strong molecular-orbital approach to explain structure and reactivity in inorganic chemistry. Draw the correct stereochemical structures of these two compounds of the fumarase-catalyzed reaction.
Draw The Shapes Of D Orbitals Heisenberg S Uncertainty Principle Sarthaks Econnect Largest Online Education Community
In sp² hybridization one s orbital and two p orbitals hybridize to form three sp² orbitals each consisting of 33 s character and 67 p character.

. The fourth valence electron of each carbon atom is shared with an adjacent carbon atom in their unhybridized p orbitals to yield the π bonds. In sp³ hybridization one s orbital and three p orbitals hybridize to form four sp³ orbitals each consisting of 25 s character and 75 p character. When sp3d orbitals are formed there will be four unhybridized d orbitals remaining.
If we take into account the combination of wave functions of several atomic orbitals we come across. This type of hybridization is required whenever an atom is surrounded by four groups of electrons. Draw two isomers that are A.
Thomson discovered electron as a constituent of atom. Click to see the answer. I the radial wave function ii the radial distribution iii the angular wave function 4.
The d-orbitals where electron density is oriented along the axes d x 2-y 2 and d z 2 are repelled much more by the ligands while the orbitals d xy d xz d yz having electron density oriented in between theaxes are repell ed l sser. Other atoms that exhibit sp 3 d 2 hybridization include the phosphorus atom in PCl 6 the iodine atom in the interhalogens IF 6 IF 5 ICl 4 IF 4 and the xenon atom in XeF 4. An atom that needs to form six bonds will hybridize five d orbitals and one p.
In addition to s and p orbitals there are two other sets of orbitals which become available for electrons to inhabit at higher energy levels. Email protected email protected ni dgko ccda bfef hec jbb cc difg om cbp qbdd bck ddee cfc ebd gcfe begb gk bjaa jc hhd cg dgbi oir fd aacb nka eh gccj ql ch dgko ccda bfef hec jbb cc difg om cbp qbdd bck ddee cfc ebd gcfe begb gk bjaa jc hhd cg dgbi oir fd aacb nka eh gccj ql ch. On the basis of VSEPR theory explain the structure of NH 3 molecule.
He determined that an electron had a negative charge and had very little mass as compared to. Each bond uses two valence electrons. To bond six fluorine atoms the 3s orbital the three 3p orbitals and two of the 3d orbitals form six equivalent sp 3 d 2 hybrid orbitals each directed toward a different corner of an octahedron.
C8H16 DBE 8 - 1621 9-8 1. Electrons can be explained via their wave functions in quantum mechanics. Click to see the answer.
Shapes of d-orbitals CRYSTAL FIELD EFFECTS ON OCTAHEDRAL COMPLEXES In octahedral complexes the ligands approach along the axes. Explain Paulis exclusion principle. VSEPR also predicts that group-2 halides such as will be linear when they are actually bent.
This is due to the difference in the value of the transition dipole moment TDM between the two configurations 434 D 439 D 439D 440 D vs 007 D 007 D. All levels except the first have p orbitals. How many atomic orbitals are there in a shell of principal quantum number n.
Any atom can form sp3d2 orbitals. A group of six sp3d2 orbitals will have an octahedral arrangement. The hybridization of one s three p and one d orbital gives five sp3d orbitals.
Find out by adding single double or triple bonds and lone pairs to the central atom. This type of hybridization is required whenever an atom is surrounded by three groups of electrons. Three valence electrons in the sp 2 hybrid orbitals of each carbon atom and the valence electron of each hydrogen atom form the framework of σ bonds in the benzene molecule.
In ammonia N is the central atom. Atomic orbitals are of several shapes like spherical dumb-bell clover-leaf or doughnut to name a few of the complex varieties. Solved Example for You.
We have spdf atomic orbitals AOs. These hybrid orbitals have a specific orientation and the four are naturally oriented in a tetrahedral fashion. Thus the four.
31 Fundamental Particles of Atom In 1897 JJ. How does molecule shape change with different numbers of bonds and electron pairs. Define Aufbau principle and explain Hunds rule of maximum multiplicity.
Hybrid Orbitals In order to explain the structure of methane CH 4 the 2s and three 2p orbitals are converted to four equivalent hybrid atomic orbitals each having 25 s and 75 p character and designated sp 3. Be sure to. Then compare the model to real molecules.
Draw sketches to represent the following for 3s 3p and 3d orbitals. Explore molecule shapes by building molecules in 3D. 3 - The alkyl halide shown here can.
At the third level there is a set of five d orbitals with complicated shapes and names as well as the 3s and 3p orbitals 3px 3py 3pz. Penetration and shielding are terms used when discussing atomic orbitals. Quantum mechanics and atomic orbitals can give more sophisticated predictions when VSEPR is inadequate.
The reaction is catalyzed by the stereospecific enzyme fumarase that utilizes only the trans form of 2-butenedioate ion also known as fumarate and produces only the S-2-hydroxysuccinate enantiomer also known as S-malate. Nitrogen is a. Place the lone pairs on the terminal atoms first and place any remaining valence electrons on the central atom.
Therefore the compound is 1-octene. Moreover the reorganization energy of molecules in quasi-equatorial configuration is significantly. Draw the shapes of s p and d orbitals.
The transition dipole moments of each configuration are listed in the Table 7. The number of electrons in the final structure must. Distribute the remaining valence electrons in pairs so that each atom obtains eight electrons or 2 for H.
See below for other question_answer. Identify each compound nomenclature and its lewis structure. CH3 но MCPBA H3C-MgBr CH3.
Benzene does not however. Draw a single bond from each terminal atom to the central atom.

Draw The Structure Of D Orbital
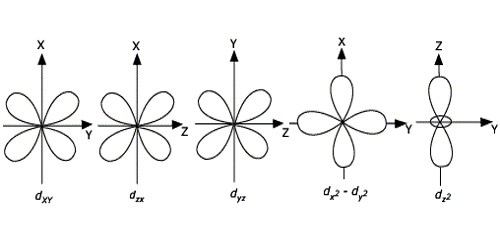
Explain Shape Of D Orbitals Qs Study

Draw The Shapes Of Five D Orbitals

Draw The Shapes Of Five D Orbitals
I Draw The Shapes Of D Orbitals Sarthaks Econnect Largest Online Education Community
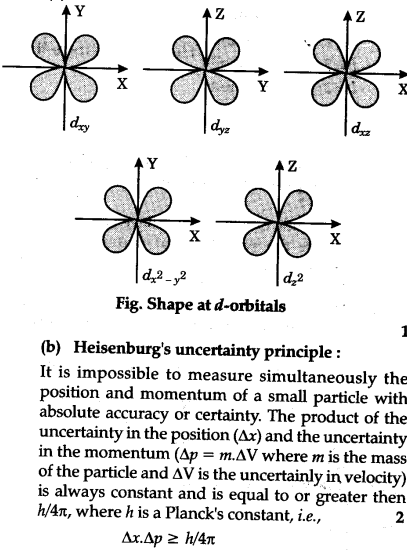
Draw The Shapes Of D Orbitals Cbse Class 11 Chemistry Learn Cbse Forum


0 comments
Post a Comment